
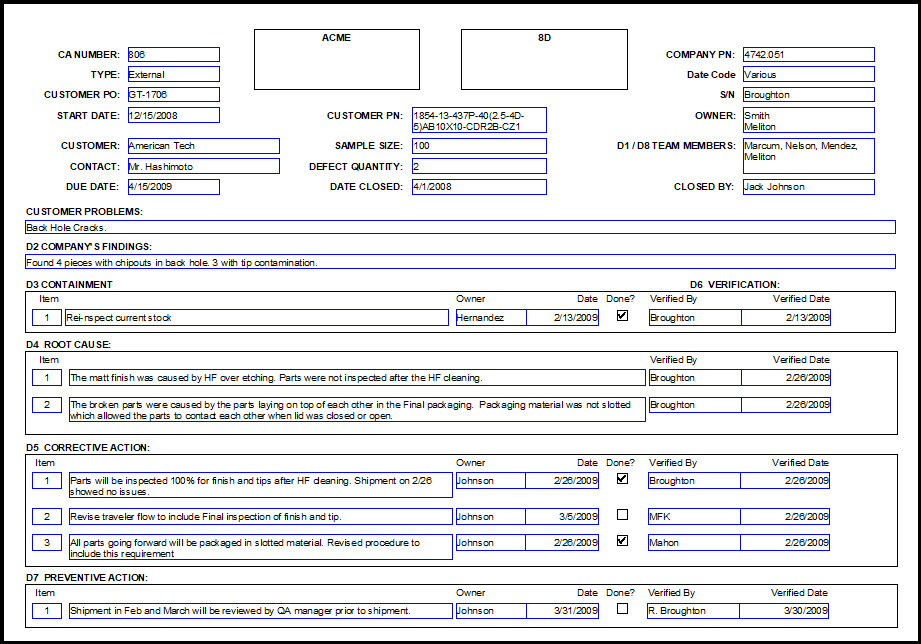
Today, clinical trial professionals are provided with guidelines, regulations, company policies, procedures, and endless amounts of training to help them with the conduct of clinical trials.ĭespite all the training one receives or all the guidelines, regulations, company policies, and procedures in place to assist with the conduct of a clinical-trial, problems including unanticipated situations or safety issues may arise (Table 1).

Actual case study illustrates some of the common problems in clinical trials.
#Corrective action preventive action how to
This article provides an overview of the root cause of these problems and how to ensure that corrective and preventive actions are addressing the actual problem rather than its symptoms. Senior Manager, Clinical Compliance, MedImmuneĬhartered MCIPD, Senior Manager, Scientific & Compliance Training, MedImmuneĪbstract : Clinical trial findings from audits reveal the same type of problems year after year despite the implementation of quality systems, compliance training, and corrective and preventive action plans.


 0 kommentar(er)
0 kommentar(er)
